Corrective and Preventive Actions CAPA Audit Trail in Lyons Quality Audit Tracking System (LQATS)
Corrective and Preventive Actions CAPA Audit Trail, specifically focusing on electronic event tracking in Lyons Quality Audit Tracking System (LQATS) related to suppliers.
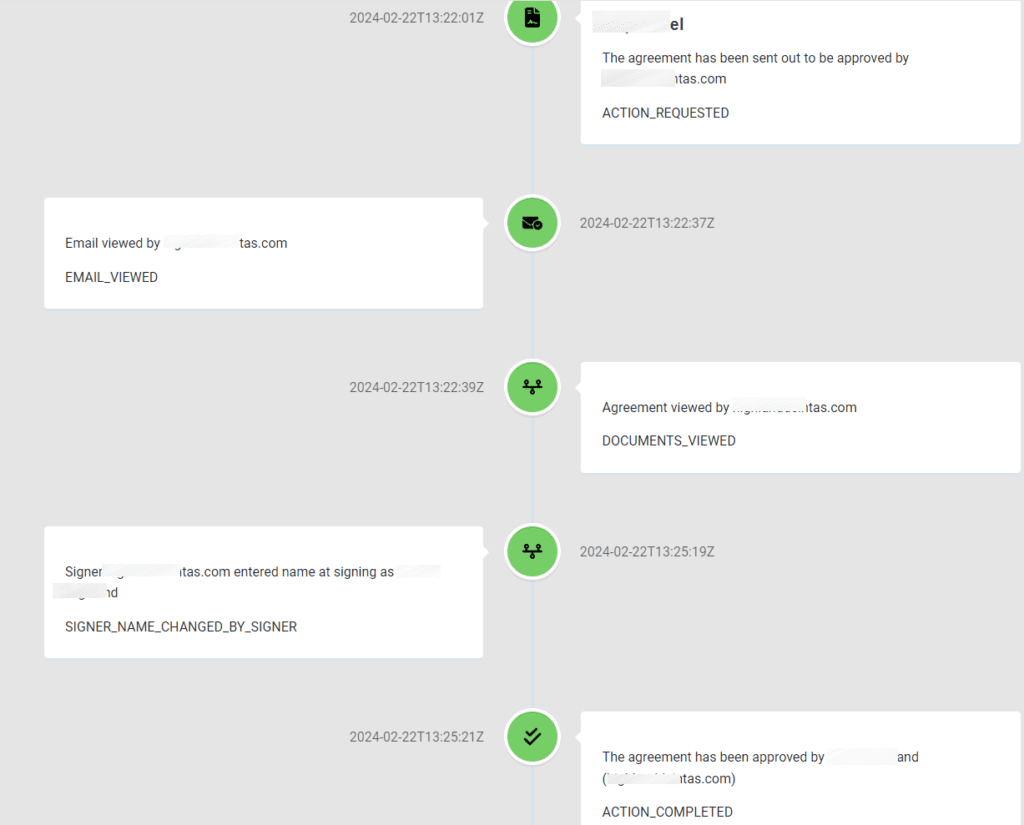
CAPA Audit Trail Definition
According to the FDA’s guidance for industry, CAPA Audit Trail is a secure, computer-generated, time-stamped electronic record that allows you to reconstruct the course of events related to the creation, modification, and deletion of an electronic record. In simpler terms, a CAPA audit trail documents the history of all actions performed on a document. It includes details such as who performed each action, when it occurred, what action was taken, and any other relevant information.
CAPA audit trails enable you to track the time-sequence development of documents, ensuring that they remain accurate and reliable without unauthorized alterations.
21 CFR Part 11 Audit Trail Requirements
The FDA’s 21 CFR Part 11 regulation mandates that systems managing electronic records must provide a secure, computer-generated, and time-stamped audit trail. This audit trail accurately records all changes made to documents. Key requirements outlined in 21 CFR Part 11, section 11.10, include:
Ensure CAPA Audit Trail Security: Only authorized individuals should access the system to make changes and sign off on documents.
Record All Activities: The audit trails must capture all relevant activities related to document creation, modification, and deletion.
Traceability: The system must allow for the reconstruction of events if an investigation is necessary.
Accuracy and Reliability: The audit trails must ensure that electronic records remain intact and unaltered.
Benefits of Implementing Audit Trails
Transparency: They provide transparency into document changes and actions taken.
Compliance: They play a crucial role in regulatory compliance, such as with 21 CFR Part 11.
Accountability: Authorized users remain accountable for their actions.
Forensic Investigation: In case of discrepancies or issues, they facilitate thorough investigations.
Quality Assurance: CAPA Audit Trail ensures the accuracy and reliability of electronic records.
An efficient electronic CAPA system enhances collaboration, accountability, and overall quality management. By embracing technology, organizations can proactively address issues and continuously improve their processes.


